Your Trusted Partner in Regulatory Affairs and Market Access for Medical Devices & IVDs in Southeast Asia Since 2013
Welcome to Andaman Medical! Whether you’re a manufacturer or distributor, our expert team is here to help you navigate the regulatory landscape and bring your products to market efficiently and compliantly.



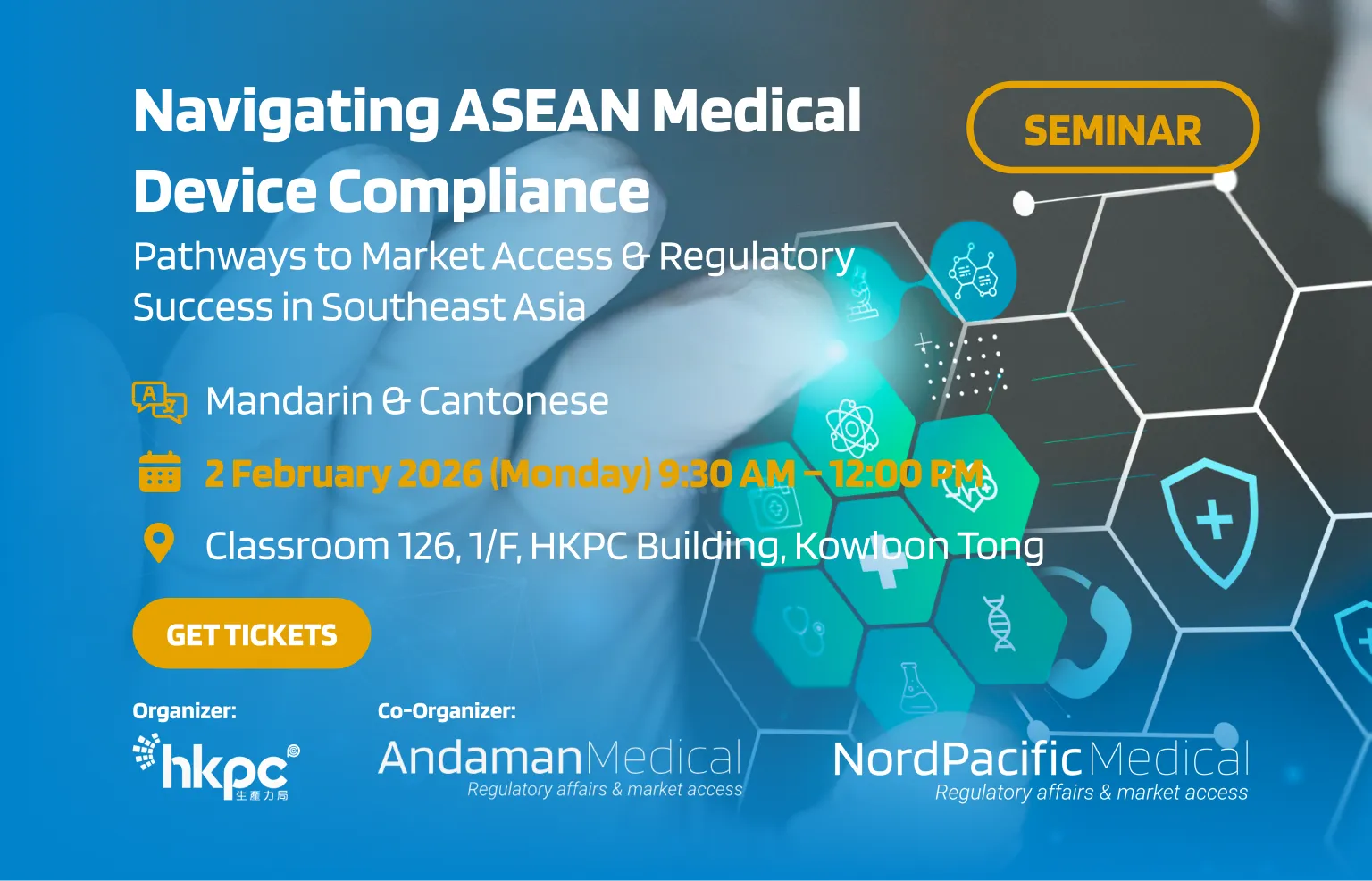
Since 2013, Andaman Medical has been transforming regulatory challenges into market opportunities across Southeast Asia. Our comprehensive solutions ensure seamless market access for medical device and IVD companies worldwide.
With eight strategic offices across Southeast Asia and additional coverage in East Asia through Nord Pacific Medical, we provide comprehensive regional support for Medical Device & IVDs Registration, Authorised Representative Services, Import Solutions and Post-Market Compliance Management.
Our Services
Expert Guidance for Regulatory Success
Medical Device & IVD Registration
Medical Device & IVD Registration
Our seasoned in-house team streamlines complex regulatory requirements, offering a one-stop solution for document preparation, submission, follow-up, and approval. This significantly reduces approval timelines while ensuring full compliance and minimizing risks.
Medical Device Compliance Consultancy
Medical Device Compliance Consultancy
Expert guidance across Southeast Asia. With deep knowledge of local regulations and global standards, Andaman Medical streamlines your product’s path from development to distribution, minimising risks and accelerating market entry.
Why Choose Us
Our expert guidance through the region’s evolving regulatory landscape ensures your product remains compliant, allowing you to bring your products to market efficiently and effectively.

Let Us Help You!
We’ve helped countless businesses navigate the regulatory process and succeed in the market—now it’s your turn!
Partner with Andaman Medical to transform regulatory complexity into your competitive advantage in Asia’s dynamic medical device market.
Start Your Journey Today
Complete the contact form or give us a call, and our team will respond within 1 working day to discuss your needs.
Guiding You Forward
We’ll schedule a consultation to understand your needs and explain how we can streamline your regulatory process.
Customized to You
We develop a detailed plan that aligns with your unique regulatory needs and market access goals.
End-to-End Support
We’ll handle everything from start to finish, ensuring your products are registered without hassle.
Who We Are
Since 2013, Andaman Medical has been transforming regulatory challenges into market opportunities across Southeast Asia. Our comprehensive solutions ensure seamless market access for medical device and IVD companies worldwide.
With eight strategic offices across Southeast Asia and additional coverage in East Asia through Nord Pacific Medical, we provide comprehensive regional support on Medical Device & IVDs Registration, Authorized Representative Services, Import Solutions and Post-Market Compliance Management.
Our Services
Medical Device & IVD Registration
Medical Device & IVD Registration
Our seasoned in-house team streamlines complex regulatory requirements, offering a one-stop solution for document preparation, submission, follow-up, and approval. This significantly reduces approval timelines while ensuring full compliance and minimizing risks.
Authorized Representative Services
Authorized Representative Services
As your dedicated authorized representative, we serve as your trusted liaison with regulatory authorities. Our proactive approach and deep regulatory expertise ensure your interests are protected throughout the process. As an independent license holder, we provide flexibility to work with different distributors, ensuring seamless transitions and full control over your licenses.
Import Solutions
Import Solutions
Let us handle the complexities of international trade regulations in the Philippines, Indonesia, and Thailand. As the license holder, we ensure efficient customs clearance and timely delivery, backed by our extensive knowledge of global trade requirements.
Post-Market Compliance Management
Post-Market Compliance Management
We assist with post-market incident reporting, including Field Safety Corrective Actions (FSCAs), mandatory problem reporting, and recalls. We monitor regulatory changes, manage reporting obligations, and implement updates, allowing you to focus on growing your business while maintaining full compliance.
Why Choose Andaman Medical as Your
Medical Device Registration Consultant in Southeast Asia

Let Us Help You!
We’ve helped countless businesses navigate the regulatory process and succeed in the market—now it’s your turn!
Partner with Andaman Medical to transform regulatory complexity into your competitive advantage in Asia’s dynamic medical device market.
Start Your Journey Today
Complete the contact form or give us a call, and our team will respond within 1 working day to discuss your needs.
Guiding You Forward
We’ll schedule a consultation to understand your needs and explain how we can streamline your regulatory process.
Customized to You
We develop a detailed plan that aligns with your unique regulatory needs and market access goals.
End-to-End Support
We’ll handle everything from start to finish, ensuring your products are registered without hassle.