ISO13485:2016 Compliance

Unwavering Commitment to Excellence



Southeast Asia Medical Device Consulting - About Us
Andaman Medical is a regulatory affairs and market access consultancy that specializes in the registration, authorized representation, importation and master distribution of medical devices and in-vitro diagnostics (IVDs) in Southeast Asia.
About Us
Andaman Medical has been a pioneer in regulatory services for medical devices and IVDs since 2013. We operate 8 offices across Southeast Asia, including Singapore, Malaysia, Indonesia, Thailand, the Philippines, Vietnam, and Cambodia. As an extension of Andaman Medical, Nord Pacific Medical operates in Hong Kong, Taiwan, Japan, and Korea, offering closer services to our clients in East Asia.
Our focus is to provide you with a hassle-free experience, allowing you to concentrate on innovating and advancing your medical devices. We ensure the seamless execution of key processes such as:
- Medical Device & IVD Registration: Our seasoned in-house team streamlines complex regulatory requirements, offering a one-stop solution for document preparation, submission, follow-up, and approval. This significantly reduces approval timelines while ensuring full compliance and minimizing risks.
- Authorized Representative Services: As your dedicated authorized representative, we serve as your trusted liaison with regulatory authorities. Our proactive approach and deep regulatory expertise ensure your interests are protected throughout the process. As an independent license holder, we provide flexibility to work with different distributors, ensuring seamless transitions and full control over your licenses.
- Import Solutions: Let us handle the complexities of international trade regulations in the Philippines, Indonesia, and Thailand. As the license holder, we ensure efficient customs clearance and timely delivery, backed by our extensive knowledge of global trade requirements.
- Post-Market Compliance Management: We assist with post-market incident reporting, including Field Safety Corrective Actions (FSCAs), mandatory problem reporting, and recalls. We monitor regulatory changes, manage reporting obligations, and implement updates, allowing you to focus on growing your business while maintaining full compliance.
Our mission
To enable medical device & IVD manufacturers and distributors to concentrate on their core business of developing and bringing medical devices & IVDs to market that improve the health and quality of life for millions. They can do this safe in the knowledge that they can rely on our dedicated Southeast Asia medical device consulting team to register, represent and import their products.
Our Values
RICE UP!
Rooted in Responsibility, driven by Integrity, united by Collaboration, and powered by Energy—our values are more than words. They are the foundation of our brand and the promise we make to every person we serve.
Responsibility
Take ownership, however it’s ok to make mistakes!
Integrity
Collaboration
Energy
Bring a level of enthusiasm and willingness to your work
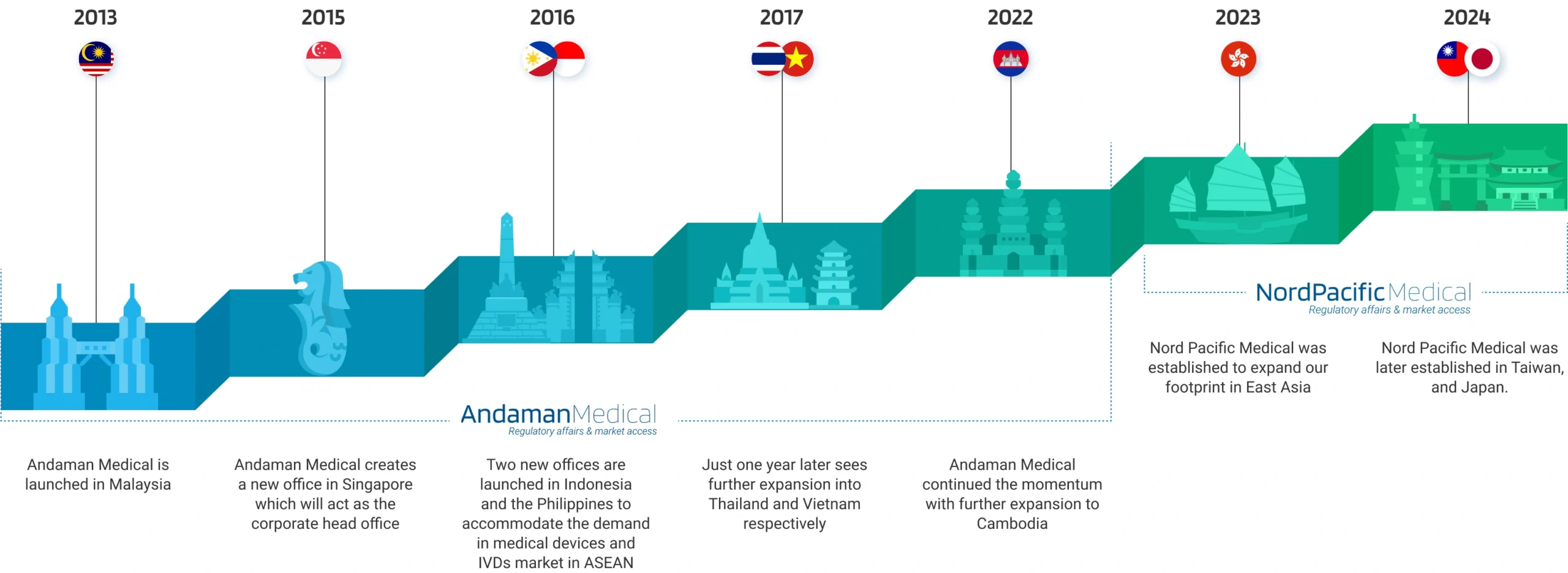
Our Milestones
Andaman Medical has been a pioneer in regulatory services for medical devices and IVDs since 2013. We operate 8 offices across Southeast Asia, including Singapore, Malaysia, Indonesia, Thailand, the Philippines, Vietnam, and Cambodia.
As we expand our footprint, Nord Pacific Medical is established in Hong Kong, Taiwan, Japan, and Korea, offering closer services to our clients in East Asia.