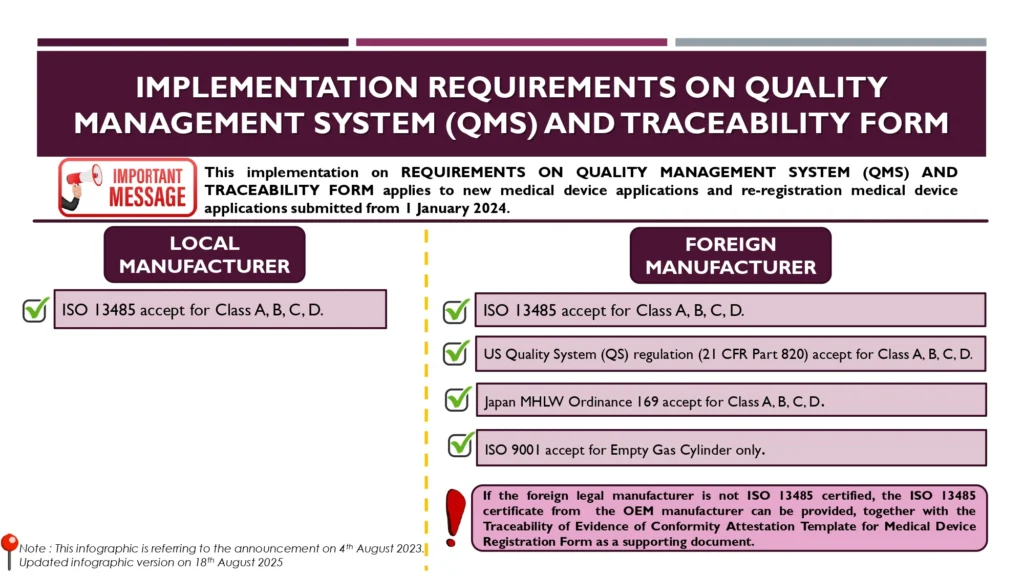
On August 18, 2025, the Medical Device Authority of Malaysia (MDA) has updated the infographic (only editorial changes) related to REQUIREMENTS ON QUALITY MANAGEMENT SYSTEM (QMS) AND TRACEABILITY FORM applies to new medical device applications and re-registration medical device applications submitted from 1 January 2024.
This infographic refers to the announcement on 4th August 2023 by MDA as per shared by Andaman Medical for the regulatory updates via website https://andamanmed.com/malaysia-mda-announces-harmonized-qms-standards-for-medical-device-registration-in-2024/ without any changes to the content.
Key Changes
- Editorial changes only; no changes to the content.
Legal Reference
Medical Device Authority. (2025, August 18). Implementation requirements on Quality Management System (QMS) and Traceability Form. Retrieved from https://portal.mda.gov.my/index.php/documents/ukk/3750-implementation-requirements-on-quality-management-system-qms-and-traceability-form/file.
Effective Date
1 January 2024
Implications to Client
Client shall take note on the Implementation requirements on Quality Management System (QMS) and Traceability Form stated in the infographic for new medical device applications and re-registration medical devices applications submitted to MDA from 1 January 2024.
Read the Infographic
You can access the official infographic here: MDA Infographic.
Next Steps
If you are preparing or submitting new medical device applications or re-registration applications in Malaysia, our regulatory team can assist you in navigating the Quality Management System (QMS) and Traceability Form requirements.
Contact us at sales@andamanmed.com or click the button below for more details.

