Local Authorised Representative in Singapore
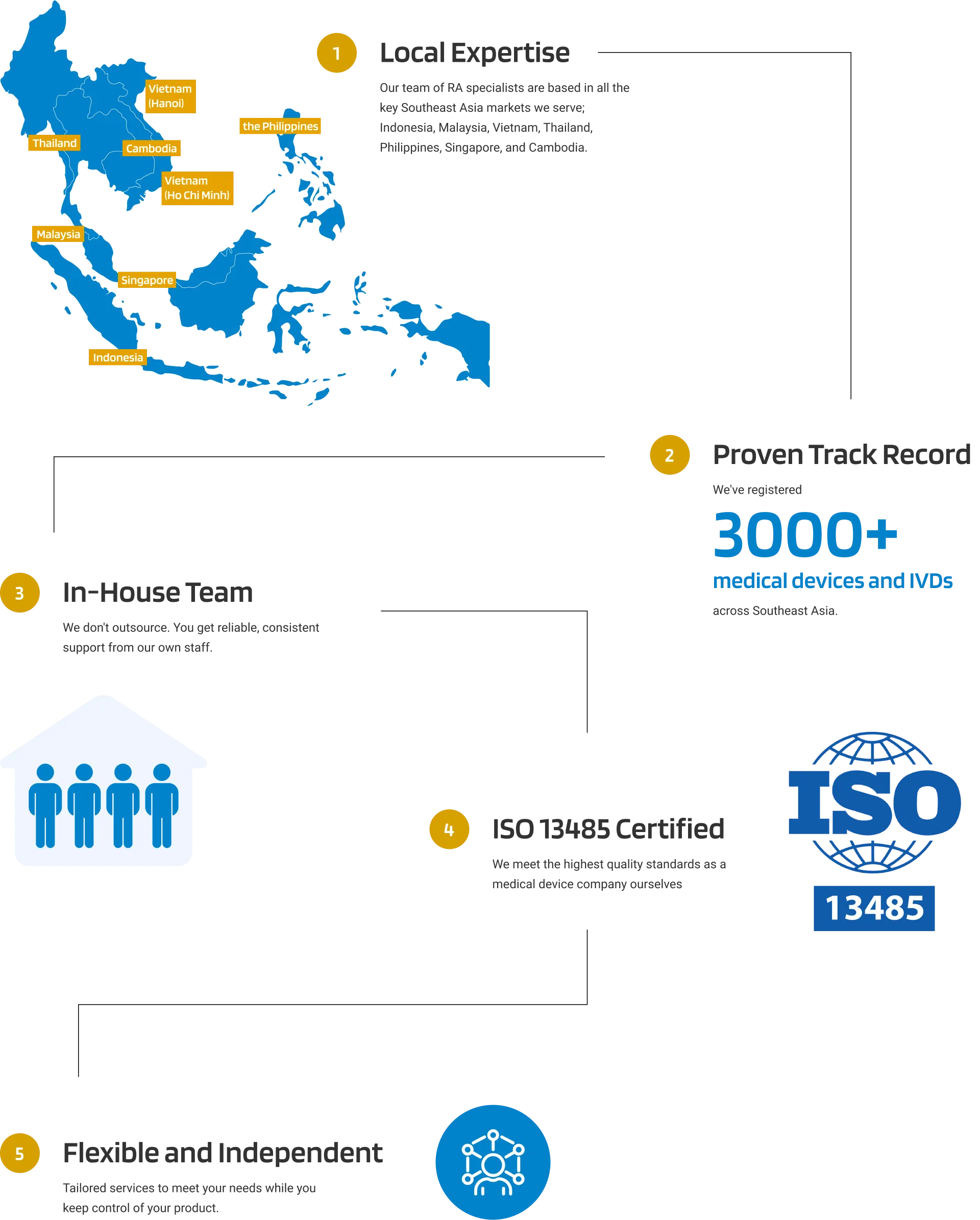
As a trusted Local Authorised Representative (LAR) in Singapore, we provide the local expertise and regulatory support you need for seamless market entry and continued compliance. We take care of the details such as submissions, notifications, and ongoing regulatory obligations so you can focus on delivering your medical innovations to healthcare providers and patients across Singapore.
Why You Need a Local Authorised Representative in Singapore
At Andaman Medical, we provide reliable and professional LAR services tailored to Singapore’s regulatory environment. With our in-depth knowledge of Health Sciences Authority (HSA) requirements, we act as your dedicated in-country representative—ensuring full compliance, managing communications with the HSA, and overseeing all necessary post-market responsibilities.
Streamlined Registration Process
Streamlined Registration Process
A LAR has the local expertise and understanding of regulatory procedures, helping to navigate complex documentation and submission requirements. This streamlines the registration process, reducing the risk of delays and rejections.
Regulatory Compliance
Regulatory Compliance
A LAR ensures that all requirements set by Singapore’s Ministry of Health are met, helping companies stay compliant with current regulations and avoid penalties or product recalls.
Efficient Communication with Authorities
Efficient Communication with Authorities
LARs act as a direct communication link between the company and Singapore’s regulatory bodies, effectively addressing any questions, requests for additional information, or changes in requirements.
Up-to-Date Knowledge of Regulatory Changes
Up-to-Date Knowledge of Regulatory Changes
LARs keep companies informed about the latest regulatory updates and policy changes in Singapore, ensuring ongoing compliance and helping to adapt strategies quickly when needed.
Faster Market Access
Faster Market Access
With the local knowledge and established relationships with regulators, a LAR can expedite the approval process, allowing faster entry into Singapore market and enabling companies to start selling their medical devices sooner.
Local Representation for Product Issues
Local Representation for Product Issues
A LAR is responsible for handling post-market surveillance, adverse event reporting, and product recalls, providing a local point of contact for regulatory authorities, which is crucial for ongoing market access.
Requirements for a Marketing Authorization Holder or Local Authorised Representative (LAR) in Singapore
CRIS Company Account and Dealer Licence
- The LAR must have a Client Registration and Identification Service (CRIS) company account from the Health Sciences Authority (HSA).
- The LAR must possess a dealer licence according to their activity, which could be a Manufacturer’s License, Registrant's Licence, Importer’s License, or Wholesaler’s License (import and export).
- Only Singaporean companies can apply for a Registrant Account.
Letter of Authorization
- A Letter of Authorization (in the prescribed format) from the foreign manufacturer is required for all products and models of devices to be registered.
- The Letter of Authorization must be issued on the Product Owner’s letterhead, with a physical signature or digital-traceable signature.
Multiple Marketing Authorisation Holders
- Yes, multiple Registrants are allowed in Singapore.
- Each Registrant must register the device individually. Each registration is treated as a new device application, and registration fees apply each time.
Process to Change Registrant
- Registrants can be changed by applying for a Change of Registrant.
- The turnaround time for this process is 40 working days.
- The HSA fee for a Change of Registrant application is S$880 per application.
Why Choose Andaman Medical for Your In-Country Representation in Thailand?
Regulatory Representation
Represent your company in all dealings with the Health Sciences Authority (HSA) for medical devices and in-vitro diagnostics.
Importers and Wholesalers Management
Add your selected Importers and/or Wholesalers for the devices to the MEDICS online portal.
Local Presence
Maintain an office in Singapore with telephone and email connectivity for efficient communication and support.
Product Registration and Notifications
- Register your product with the HSA.
- Submit change notifications as required.
- Transfer product licences when necessary.
Conformity Assessment
Ensure that the appropriate conformity assessment procedure has been carried out, if required.
Complaint Handling
Assist with handling complaints related to your products.
Post-Market Surveillance
- Ensure post-market surveillance once your product is authorised and placed on the market.
- Report Adverse Events and Field Safety Corrective Actions.
HSA Liaison
- Accept all calls from the HSA regarding your company and its products.
- Liaise with your company to address inquiries or schedule inspections of manufacturing facilities, if required.
Regulatory Monitoring
Monitor regulations and alert you to any changes or new regulations applicable to your product.