The Thai Ministry of Public Health has issued a new Notification titled Principles, Methods, and Conditions for Displaying Labels and Medical Device Package Inserts, B.E. 2568 (2025). This Notification replaces the previous 2020 regulation and introduces streamlined labeling rules, harmonized package insert requirements for home-use and professional-use devices, updated provisions for software and special device categories, and a shorter timeframe for completing import labeling.
The new requirements will take effect on June 20, 2026, with a two-year transition period allowing continued use of labels approved under the 2020 Notification until June 20, 2028.
Thai FDA Official Website: กองควบคุมเครื่องมือแพทย์
Key Changes at a Glance
Notification B.E. 2563 (2020) | Notification B.E. 2568 (2025) – New | |
Label |
|
|
Package Insert |
|
|
Specific Rules for Certain Device Types |
|
|
Import Labeling at Checkpoint |
|
|
Purpose of the New Notification
The updated regulation aims to:
- Modernize and simplify labeling requirements for medical devices marketed in Thailand.
- Harmonize package insert requirements across device categories, reducing differing obligations for home-use and professional-use products.
- Introduce targeted rules for software applications, reusable instruments, and accessories.
- Shorten the timeline for completing import labeling at customs checkpoints.
- Improve consistency, clarity, and regulatory oversight to support safe device use and accurate communication to users.
Scope of Application
This Notification applies to all medical devices requiring labels and package inserts, including:
- Home-use and professional-use medical devices
- Software-based medical devices (including applications with physical forms)
- Reusable surgical and dental instruments
- Accessories supplied with medical devices
- Devices undergoing importation and inspection at Thai customs
The regulation provides updated, consolidated requirements for labeling content, display format, and acceptable media (paper or electronic IFUs).
Annex A: English Translation of Label Infographics
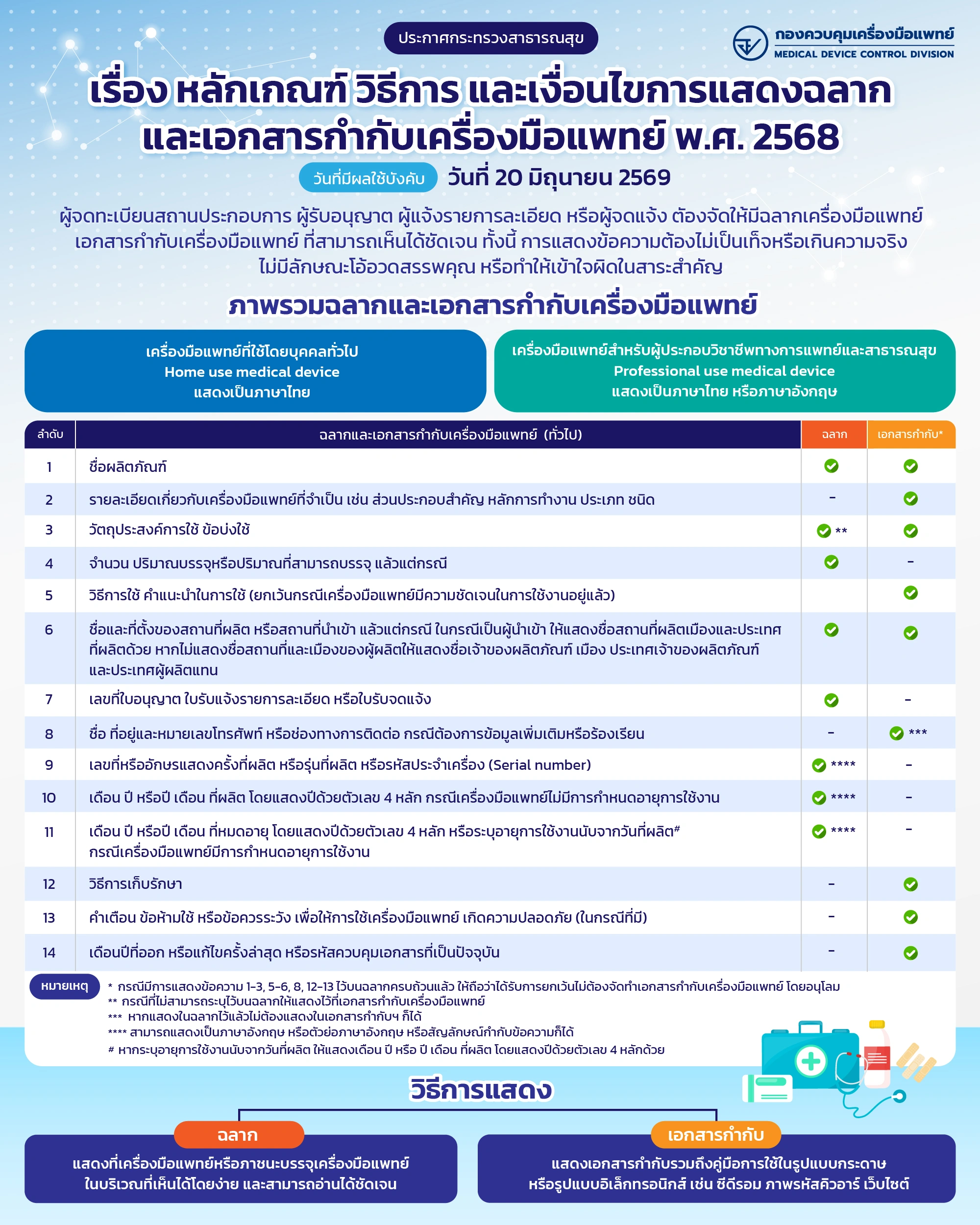
Overview of Labels and Medical Device Package Inserts
Medical device labels and package inserts must be clearly visible, accurate, non-misleading, and must not exaggerate or claim properties beyond actual performance.
- Home use medical device must be labeled in Thai (English is optional).
- Professional use medical device must be labeled in English and/or Thai.
No. | Labels and Medical device package inserts (in general) | Label | Package Insert* |
1 | Product name | ✓ | ✓ |
2 | Necessary details relating to the medical device, such as main components, operation principles, category and type | – | ✓ |
3 | Intended uses or Indications | ✓ ** | ✓ |
4 | Number, quantity contained or quantity that can be contained, as the case may be | ✓ | – |
5 | Instructions for use (except for a medical device of which usage is already apparent) | – | ✓ |
6 | Name and location of the local manufacturing facility or import facility depend on type of establishment license.
| ✓ | ✓ |
7 | Reference number of the license, notification number or listing number | ✓ | – |
8 | Name, address and telephone number, or contact information in cases of requesting additional information or making complaints | – | ✓ *** |
9 | Number or alphabet identifying the batch number or lot number, or serial number | ✓ **** | – |
10 | Month and year or year and month of manufacturing which year shall be displayed in a four-digit number, in the case where the medical device does not have an expiration date. | ✓ **** | – |
11 | Month and year or year and month of expiry which year shall be displayed in a four-digit number or specify the service life calculated from the date of manufacture, in the case where the medical device has a specified service life. | ✓ **** | – |
12 | Storage instructions | – | ✓ |
13 | Warnings, contraindications or precautions for safe usage of the medical device (if any) | – | ✓ |
14 | Month and year of issuance or latest revision of the medical device package insert, or up-to-date control code of the document | – | ✓ |
Legend:
* Case of displaying information items 1-3, 5-6, 8, 12-13 has been fully displayed on the label, it shall be deemed exempt from the requirement to prepare medical device package insert.
** Where the information cannot be indicated on the label, it shall be stated in the medical device package insert.
*** If the information is already displayed on the label, it is not required to be included in the medical device package insert.
**** It may be presented in English, in English abbreviations, or by using symbols accompanying the text.
If the service life is specified as calculated from the date of manufacture, the month and year or the year and month of manufacture shall be indicated, with the year shown in four-digit number.
How to display
- Label: Displaying on medical devices or packaging of medical devices that can be easily seen and clearly read.
- Medical device package insert: The accompanying documentation, including the instructions for use, may be provided in paper form or in electronic form, such as CD-ROMs, QR codes, or websites.
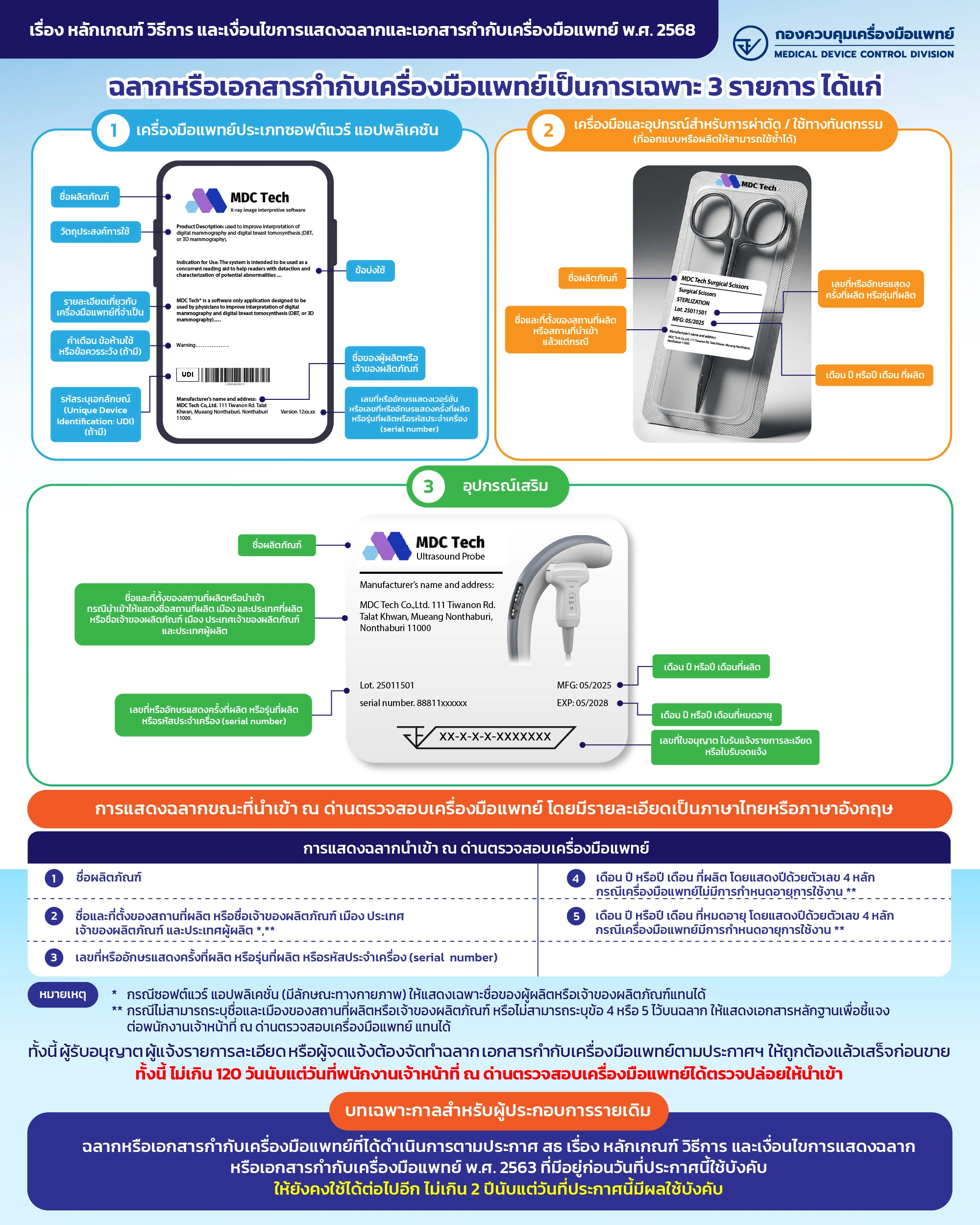
Special Labels and Medical Device Package Inserts 3 Products
Implications to Clients and Stakeholders
The new Notification represents a significant modernization of Thailand’s labeling and IFU framework. Stakeholders should prepare to demonstrate:
- Updated labeling content aligned with the revised 9-item requirements
- Harmonized IFUs for home-use and professional-use devices
- Compliance with new rules for software, reusable instruments, and accessories
- Earlier completion of import labeling (within 120 days)
- Documented procedures for label/IFU updates during the transition period
Early gap assessments and transition planning are recommended to avoid disruption to manufacturing, importation, and market supply. Organizations should also review electronic IFU formats and ensure supporting documentation for customs inspections is readily available.
This update is provided for regulatory awareness and operational planning.
Contact Information
For inquiries or support regarding medical device labeling, package insert requirements, or regulatory compliance in Thailand under Notification B.E. 2568 (2025), please contact: sales@andamanmed.com

