Medical Device Post-Market Surveillance in Thailand
Maintaining compliance, safety, and reliability for your medical devices in Thailand can be complex. That’s where Andaman Medical steps in.
With our expert post-market surveillance services in Thailand, we manage the entire process—from meeting Thai FDA requirements to tracking ongoing product performance and safety. We help ensure your devices remain compliant, trusted, and ready for long-term success in the Thai market.
Why Get Medical Devices PMS in Thailand?
Post-market surveillance goes beyond regulatory compliance. It safeguards patient safety, ensures continued market access, and reinforces confidence in your brand.
At Andaman Medical, we provide end-to-end post-market surveillance (PMS) solutions designed specifically for Thailand’s regulatory framework. With our in-depth knowledge of Thai FDA requirements and the medical device industry, we help you maintain compliance, manage risks proactively, and enhance product quality—all while supporting better health outcomes.
Here’s how Andaman Medical supports your post-market surveillance needs in Thailand:
Regulatory Compliance
Regulatory Compliance
PMS ensures that your products meet ongoing regulatory requirements set by the Thai FDA, avoiding penalties or market withdrawal.
Patient Safety
Patient Safety
Monitoring product performance helps identify and address safety issues early, protecting patients from potential harm.
Risk Management
Risk Management
PMS allows you to detect and mitigate risks associated with device use, reducing the likelihood of adverse events.
Market Credibility
Market Credibility
Ongoing surveillance demonstrates your commitment to product safety and quality, enhancing your reputation among healthcare providers and patients.
Improved Product Performance
Improved Product Performance
Feedback from PMS can be used to improve device design and functionality, enhancing overall product quality.
Quick Response to Issues
Quick Response to Issues
PMS enables rapid response to product defects or failures, minimising the impact on patients and your market position.
Sustained Market Access
Sustained Market Access
Regular surveillance helps maintain compliance, ensuring your devices continue to be sold legally in Thailand.
Medical Device PMS Requirements in Thailand
As part of post-market surveillance of medical devices, Thailand requires the reporting of medical device defects or adverse events (AEs) affecting any person, whether the incident occurs within Thailand or abroad, when the medical device is suspected to be a contributory cause. The reporting is required for the following criteria:
- serious threat to public health incidents
- death or serious injury
- if scientific data or evidence indicates that the incident can lead to death or serious harm to consumers if it were to recur.
In order to reduce or eliminate the risks associated with the medical device defect or AE which can result in a serious threat to public health or imminent risk of death or serious harm, the surveillance and reporting of a FSCA is required when the Product Owner undertakes any of the following actions either in Thailand or outside Thailand:
- product recall
- device modification
- product exchange
- device destruction
- safety notification updates and changes.
In order for the Thailand’s FDA to adequately evaluate the cause of the medical device defect or AE, and the subsequent FSCAs, the following information is required as a minimum for the reporting:
- description of the device defect or AE, and FSCA
- place where the device defect or Adverse Effect occurred
- persons affected by the incident (only for AE)
- health hazard evaluation report or any related documents, together with the initial report (only for FSCA).
The Adverse Effect and FSCA reports can be submitted via the online Medical Device Problem Reporting System on the Health Product Vigilance Centre website.
Timelines for AE and FSCA reporting in Thailand
| Type of Report | Timeline | |
|---|---|---|
| 1.1 Adverse Event in Domestic Case | Initial report | Serious threat to public health: Immediately or not later than 48 hours |
| Death or serious injury: Immediately or within 10 days | ||
| Potential to cause death or serious injury if the event recurs: Within 30 days | ||
| Follow-up report | 30 days from initial report | |
| 1.2 Adverse Event in Foreign Case | Report | Twice a year< (1) Incidents occurring from January to June should be reported within the month of August. (2) Incidents occurring from July to December should be reported within the month of February of the following year |
| 1.2. FSCA in Thailand and overseas Domestic Case & Foreign Case | Initial report | Within 48 hours of implementation of the FSCA |
| Follow-up report | Within 21 days of the previous report |
Post Market Surveillance of Medical Devices in Thailand
The Thai Food and Drug Administration (Thai FDA) as a department of the Ministry of Public Health requires the Establishment License Holder, the Product License Holder or the Specification Provider to report medical device problems including device defects or Adverse Effect (commonly known as Adverse Events AE) and Field Safety Corrective Actions (FSCA).
Post Market Surveillance in Thailand is regulated under B.E. 2559 (2016): Notification of Criteria, Procedures and Requirements on Reporting Medical Device Defects or Adverse Effects Occurring to Consumers and Reporting of Field Safety Corrective Actions. As a member country of ASEAN, this regulation aligns with the control of medical devices in the ASEAN Medical Device Directive (AMDD) which came into effect on 1st January 2015. Please read on to understand how post market surveillance is implemented in Thailand.
What are the requirements for medical device PMS in Thailand?
Thailand requires the reporting of device defects or AEs happening to any person who is affected by the device either in Thailand or outside Thailand when the medical device is suspected to be a contributory cause of the incident, for the following criteria:
- serious threat to public health incidents
- death or serious injury
- if scientific data or evidence indicates that the incident can lead to death or serious harm to consumers if it were to recur.
In order to reduce or eliminate the risks associated with the device defect or AE which can result in a serious threat to public health or imminent risk of death or serious harm, the reporting of a FSCA is required when the Product Owner undertakes any of the following actions either in Thailand or outside Thailand:
- product recall
- device modification
- product exchange
- device destruction
- safety notification updates and changes.
In order for the Thai FDA to adequately evaluate the cause of the device defect or AE, and the subsequent FSCAs, the following information is required as a minimum for the reporting:
- description of the device defect or AE, and FSCA
- place where the device defect or Adverse Effect occurred
- persons affected by the incident (only for AE)
- health hazard evaluation report or any related documents, together with the initial report (only for FSCA).
The Adverse Effect and FSCA reports can be submitted via the online Medical Device Problem Reporting System on the Health Product Vigilance Center website.
What are the timelines for AE and FSCA reporting in Thailand?
The Thai FDA stipulates timelines for reporting of the incident or FSCA as below:
| Type of Report | Timeline | |
|---|---|---|
| Domestic Case | Initial report | Serious threat to public health: Immediately or not later than 48 hours |
| Death or serious injury: Immediately or within 10 days | ||
| Potential to cause death or serious injury if the event recurs: Within 30 days | ||
| Follow-up report | 30 days from initial report | |
| Foreign Case | Report | Twice a year |
| Domestic Case & Foreign Case | Initial report | Within 48 hours of implementation of the FSCA |
| Follow-up report | Within 21 days of the previous report |
How Andaman Medical can help you with post market surveillance in Thailand
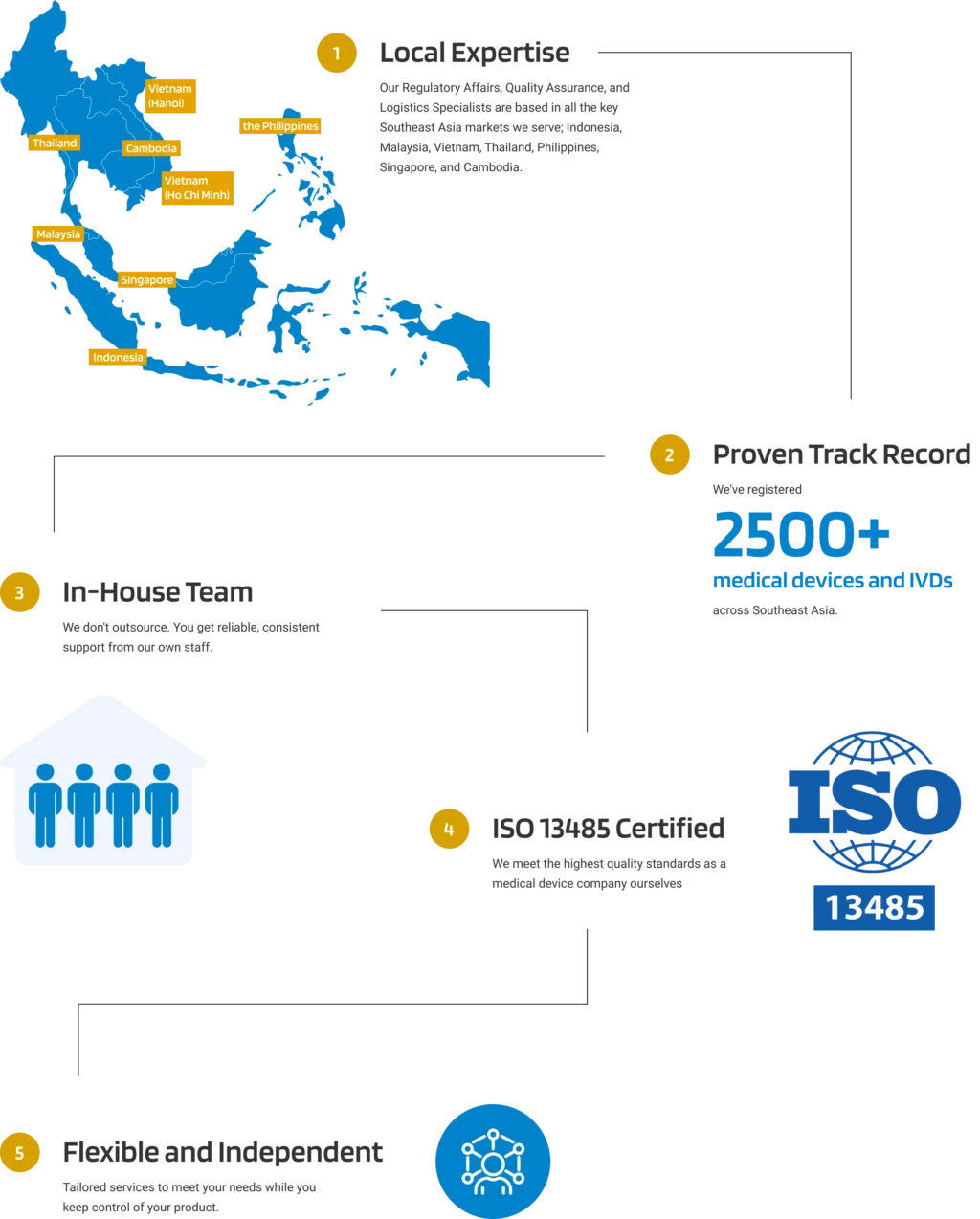
With six local offices throughout Southeast Asia, Andaman Medical provides support for your post-market surveillance activities including adverse effect and FSCA mandatory reporting. Our local, in-house staff liaise with the Thai FDA to help you maintain compliance once your medical device is placed on the market. Our services include:
- support for your mandatory reporting of Adverse Effects and Field Safety Corrective Actions
- identify requirements in medical device directives, standards, and guidance documents to ensure effective implementation of a post-market surveillance system
- evaluate your PMS data to ensure that existing processes and outputs are fully compliant
- monitor and report on any regulatory changes to ensure ongoing compliance